
Back at the start of the year in our Smartphone Futurology series, we discussed the technology behind the battery in smartphones and what's to come in the future. This article is a quick update to that piece, looking at some of the recent developments in batteries based on Lithium chemistry — like the ones powering the vast majority of smartphones.
We'll take a closer look at what reduces your phone's battery life over time, and how high-capacity technologies like Lithium Sulfur batteries and Lithium metal anodes are closer than ever to becoming practical. Join us after the break.
Read More: The latest breakthroughs in phone battery technology
Why your battery's capacity decreases over time

Image credit: Joint Center for Energy Storage Research
A group led by the Joint Centre for Energy Storage Research in the US managed to gather evidence on the processes behind the deterioration of lithium batteries over time[1]. In my original article, I mentioned the dendritic (branching like a tree) growths on lithium metal anodes over time reducing battery capacity.
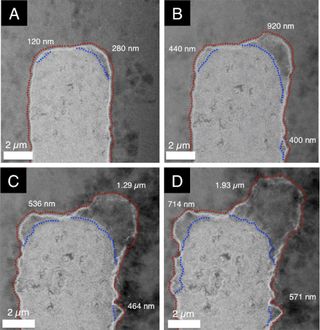
Lithium metal deposition on Li-po electrode over time
Credit: Joint Center for Energy Storage Research
Get the Windows Central Newsletter
All the latest news, reviews, and guides for Windows and Xbox diehards.
The team developed a new method using STEM (scanning transmission electron microscopy - a method for analysing incredibly small structures) to observe these deposits in a lithium polymer battery over time.
The anode of a lithium battery is what determines the total capacity, and these growths disrupt how efficiently the anode is able to store lithium ions and thus reduce the battery's capacity. It's also been shown that these dendritic growths of lithium metal can be dangerous and cause internal failures which lead to the battery ballooning, or even worse, exploding[2].
With these breakthrough abilities to observe such processes, the team have been able to determine the factors which control these growths which will help researchers in the field to improve the longevity and safety of commercial lithium based batteries.
Improvements in Lithium-Sulfur
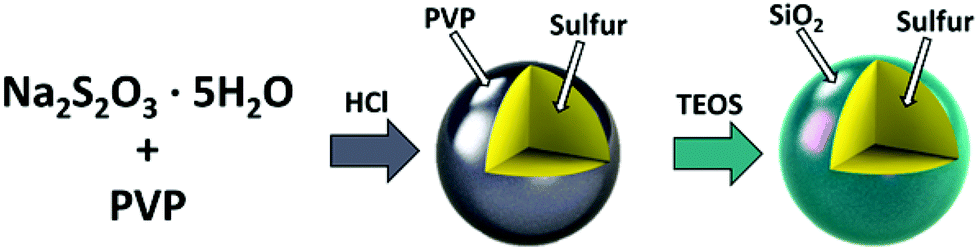
Image credit: University of California
There has been a dramatic increase in number of published papers on lithium sulphur technology, and as explained previously the technology is viewed as the next iteration in lithium battery technology, replacing the widely adopted lithium polymer cells. To recap:
Lithium-sulfur is an extremely attractive replacement for current technologies as it's just as easy to produce, has a higher charging capacity. Better still, it doesn't require highly volatile solvents which drastically reduce the risk of fire from shorting and punctures.
More on Lithium-sulfur and other future battery technologies
Recently, a group from the University of California have solved one of the issues surrounding lithium-sulphur chemistry, publishing a paper on it last month[3].
As issues with the longevity of Li-S batteries are solved, the technology moves further towards being a practical reality.
During the chemical reactions that occur in the charge and discharge processes, polysulfide chains are formed. These chains must flow through the electrolyte intact and this is where the issue lies, the polysulfide can sometimes dissolve into the solution[4, 5] and greatly impacts battery longevity.
The group developed a method of coating these polysulfides into nanospheres using a thin layer of silicon dioxide (essentially glass), which keeps the polysulfide away from the electrolyte while being able to move easily through it between the electrodes. With issues like these being constantly solved by numerous hard working research groups, the future of lithium-sulfur batteries being in our phones edges closer every single day.
Lithium Metal Anodes coming to fruition

Image credit: SolidEnergy Systems
If you remember from the battery futurology article, I mentioned how being able to use lithium metal as the anode is the "holy grail" of anode materials due to the extra capacity they bring.
SolidEnergy Systems Corp. have been showing off their "anodeless" lithium battery, which essentially replaces the normal graphite and composite anodes with a thin lithium metal anode. They claim they double the energy density compared to a graphite anode and 50% compared to a silicon composite anode.
The latest 'anodeless' batteries claim to double the energy density of what's in your phone right now.

The above image which SolidEnergy have published helps show the drastic reduction in size, though I should mention it's slightly misleading. Both the Xiaomi and Samsung batteries are designed to be replaceable, so would have an additional plastic shell and additional electronics such as a charging circuit or even (in some Samsung batteries) an NFC antenna.
However, having said that, you can see the substantial size difference between the iPhone's 1.8 Ah internal battery and the 2.0 Ah SolidEnergy battery pack in the BBC's news report.
What it all means

With several manufacturers' flagship phones pushing towards thinner designs, the need for denser batteries is becoming even greater. Cramming more battery power into a smaller area also opens up the possibility of getting several days of use out of larger "phablet" style handsets, while providing more juice for the power-hungry processors of the future.
We're looking at a future where it'll be easier than ever to avoid the dreaded dead smartphone battery.
And when it comes to lithium-sulfur batteries, the reduced risk of fire from shorting out or puncturing should make our devices safer to use, and less dangerous (and costly) to for manufacturers to transport.
Combine this with recent progress towards faster charging and the growth of wireless charging in recent years, and we're looking at a future where it'll be easier than ever to avoid a dead smartphone battery.
So when will we start seeing these new technologies becoming available? SolidEnergy estimates its "anodeless" solution will hit the market in 2016, and we're looking at a similar timetable for Li-S batteries as well, given the recent developments around this technology. That's not to say they'll be shipping in actual mobile devices in the next year — nevertheless, the revolution in battery technology we've all been waiting for can't be far away.
More Futurology: Read about the future of smartphone tech{.large .cta}
References
- B.L. Mehdi, J. Qian, E. Nasybulin, C. Park, D.A. Welch, R. Faller, H. Mehta, W.A. Henderson, W. Xu, C.M. Wang, J.E. Evans, J. Liu, J.G. Zhang, K.T. Mueller, and N.D. Browning, Observation and Quantification of Nanoscale Processes in Lithium Batteries by Operando Electrochemical (S)TEM, Nano Letters, 2015. 15(3): p. 2168-2173.
- G. Zheng, S.W. Lee, Z. Liang, H.-W. Lee, K. Yan, H. Yao, H. Wang, W. Li, S. Chu, and Y. Cui, Interconnected hollow carbon nanospheres for stable lithium metal anodes, Nat Nano, 2014. 9(8): p. 618-623.
- B. Campbell, J. Bell, H. Hosseini Bay, Z. Favors, R. Ionescu, C.S. Ozkan, and M. Ozkan, SiO2-coated sulfur particles with mildly reduced graphene oxide as a cathode material for lithium-sulfur batteries, Nanoscale, 2015.
- Y. Yang, G. Zheng, and Y. Cui, Nanostructured sulfur cathodes, Chemical Society Reviews, 2013. 42(7): p. 3018-3032.
- W. Li, Q. Zhang, G. Zheng, Z.W. Seh, H. Yao, and Y. Cui, Understanding the Role of Different Conductive Polymers in Improving the Nanostructured Sulfur Cathode Performance, Nano Letters, 2013. 13(11): p. 5534-5540.

